Abstract
BACKGROUND: Ponatinib is a third generation tyrosine kinase inhibitor (TKI) used to treat resistant chronic myeloid leukemia (CML). It has been associated with cardiovascular adverse events (CAEs); most specifically hypertension and arterial thrombotic events. Most CAEs are dose-dependent. The frequency of CAEs in the real-world setting has not been previously described. In this study, we describe the survival outcomes and associated toxicities in ponatinib-treated patients at Moffitt Cancer Center (MCC).
METHODS: A retrospective review of CML patients treated with ponatinib at MCC between years 2011 and 2017 was performed to identify patients treated with ponatinib. Demographics, disease-specific variables and clinical outcomes were collected in accordance with the Institutional Board Review approved protocol. Frequency of adverse events on ponatinib were categorized. Pearson's chi-square test for independence was used to determine significance for categorical variables. Kaplan-Meier analysis with log-rank test was performed to estimate overall survival (OS).
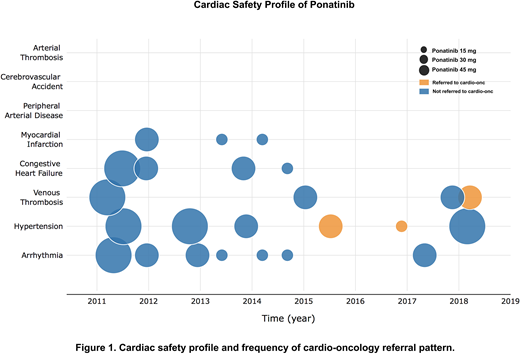
RESULTS: A total of 78 patients (38 females/40 males) treated with ponatinib with median age of 42.5 (range 14-70) years were included in the analysis. At the time of ponatinib initiation, 51 patients were in chronic phase (CP), 9 in accelerated phase (AP), and 18 in blast phase (BP). Ponatinib was 3rd or 4th line therapy in 65% of CP patients, with a mean starting dose of 39.65 mg per day. Dose reduction occurred in 64.7% of patients with CP and the mean duration of ponatinib was 14.6 months. The most common non-cardiovascular adverse events (NCAEs) were thrombocytopenia (39.7%), abdominal pain (33.3%), anemia (28.2%), elevated lipase (28.3%), and rash (26.9%) in a dose-dependent fashion. Most common new onset CAEs included arrhythmia (9%) and hypertension (7.7%). Three patients (3.8%) experienced a myocardial infarction, and two of these had significant cardiovascular risk factors at baseline. No other arterial thrombotic events were reported. A total of 18 patients (23.1%) experienced some form of CAE. Prior to 2014, more patients were placed on 45 mg ponatinib and more CAEs occurred during that time. After 2014, there is an increased awareness of potential cardiotoxicity associated with ponatinib and referral to cardio-oncology (CO) became a routine practice in our institution. Figure 1 summarizes the cardiac safety profile of ponatinib and frequency of CO referral. Ponatinib 45 mg and CAEs are not independent (X2 (1, N = 82) = 7.68, p < 0.05), but this correlation was lost when testing CAEs against patients who were on ponatinib 45 mg and either referred to CO or on cardiac protective medications (X2 (1, N = 82) = 3.55, p > 0.05) suggesting CAEs become independent of ponatinib dosing when cardioprotective measures are put in place. In patients with CP-CML, 69.6% achieved a complete cytogenetic response (CCyR) and 58.7% had a major molecular response (MMR). Patients with AP and BP achieved CCyR in 37.5% and 68.8%, respectively. Among the BP patients who achieved at least CCyR (n = 11), 81.1% of the patients had received TKI with chemotherapy at the time of BP. Five-year median OS of the entire cohort was 63%. With a median follow up of 32.5 months, the median OS from ponatinib initiation was not reached (NR) in CP with allogeneic stem cell transplantation (allo-SCT) (n = 17) or without allo-SCT (n = 34); was 32.9 months in AP with allo-SCT (n = 4), NR in AP without allo-SCT (n = 5); and NR in BP with allo-SCT (n = 6), and 5.45 months in BP without allo-SCT (n = 12) (p < 0.0001).
CONCLUSIONS: In our series, the incidence of new arterial thrombotic events and hypertension appears lower than reported in the PACE trial. The incidence of CAEs declined after 2014 when early dose reductions became standard practice and early referrals to cardio-oncology increased. Non-CAEs and outcomes were similar to previous reports. Limitations of this analysis include its retrospective nature and small sample size. Future studies analyzing the impact of specific interventions on mitigating cardiovascular events are needed.
Sweet:BMS: Honoraria; Novartis: Consultancy, Honoraria, Speakers Bureau; Celgene: Honoraria, Speakers Bureau; Astellas: Consultancy; Agios: Consultancy; BMS: Honoraria; Agios: Consultancy; Astellas: Consultancy; Phizer: Consultancy; Jazz: Speakers Bureau; Phizer: Consultancy; Celgene: Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau; Jazz: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal